Study Guide
Field 004: Chemistry
Sample Selected-Response Questions
Recommendation for individuals using a screenreader: please set your punctuation settings to "most."
General Test Directions
This test contains two sections: (1) a section with selected-response questions and (2) a constructed-response section. The directions for the constructed-response assignment appear before that section.
Each question in the first section is a selected-response question with four answer choices. Read each question and answer choice carefully and choose the start uppercase ONE end uppercase best answer. Try to answer all questions. In general, if you have some knowledge about a question, it is better to try to answer it. You will start uppercase NOT end uppercase be penalized for guessing.
A calculator is available to you for this test. To access the calculator, click on the
icon located in the upper left corner of the screen. A pop-up window containing the calculator will appear. You can reposition the calculator by placing your cursor in the blue area above the calculator and dragging the window to the location of your choice.
Use the numbers on the keyboard and/or point and click with the mouse to enter your computations into the on-screen calculator. When you are finished, close the calculator by clicking the
button in the upper right corner of the calculator.
Reference materials will also be available to you during this test. To access these reference materials, click on the
icon located in the lower left corner of the screen.
You may work on and complete the selected-response questions and the constructed-response assignment in any order that you choose. Be sure to allocate your time carefully so that you are able to complete the entire test within the testing session.
Use of any other type of calculator or outside reference materials during the testing session is prohibited.
Sample Selected-Response Questions
Competency 0002
Analyze the historical progression of scientific knowledge and the role of science
in contemporary society.
1. In the late eighteenth century, Antoine and Marie Lavoisier conducted a series of experiments involving combustion reactions in sealed glass containers. These experiments were significant primarily because they were the first to document that:
- atoms of different elements are present in fixed proportions in a given compound.
- combustion involves the splitting of molecules and the recombination of the atoms into different molecules.
- the total mass of the products of a reaction equals the total mass of the original substances.
- the elemental identities of individual atoms are not changed during a chemical reaction.
correct response: c. this question requires the examinee to demonstrate knowledge of the significance of key experiments and individuals in the history of chemistry. the experiments of the lavoisiers demonstrated that the total mass of the products of a reaction equals the mass of the reactants. these experiments were critical in demonstrating the principle of the conservation of matter.
Competency 0003
Apply knowledge of the crosscutting concepts in the sciences.
2. In which of the following natural systems is the scale of the system most critical to its proper functioning?
- the bones and muscles in the human arm that together operate as a third-class lever
- a single-celled organism that uses diffusion to exchange substances with its environment
- the side-to-side undulating motion of a snake that allows it to move on land and in water
- a turtle's hard upper shell that protects it from predators and environmental stresses
correct response: b. this question requires the examinee to demonstrate the ability to apply the concepts of scale to the analysis of scientific phenomena. diffusion is a spontaneous process in which molecules or ions move from an area of high concentration to one of low concentration. in single-celled organisms, the movement occurs across a semi-permeable membrane. the size of a cell, particularly its surface area to volume ratio, has a significant effect on whether or not diffusion will occur rapidly enough to sustain the metabolic needs of the organism.
Competency 0005
Apply knowledge of the periodic table.
3. start bold Use the diagram below to answer the question that follows. end bold
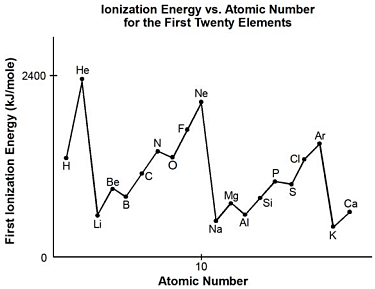
The diagram is a graph with the title Ionization Energy versus Atomic Number for the First Twenty Elements. The vertical axis is labeled First Ionization Energy in kJ per mole, with values marked at 0 and 2400. The horizontal axis is labeled Atomic Number, with values marked at 0 at the origin and 10 at the midway point. The data points form a roughly sawtoothed pattern, with hydrogen at about 1400 and helium at about 2400 forming the first peak. The next eight elements are at about 550 for lithium, 900 for beryllium, 800 for boron, 1100 for carbon, 1300 for nitrogen, 1600 for oxygen, 1900 for flourine, and 2100 for neon. The next eight elements are at about 500 for sodium, 800 for magnesium, 600 for aluminum, 800 for silicon, 1000 for phosphorus, 1200 for sulfur, 1400 for chlorine, and 1600 for argon. The last two elements that appear on the graph are at about 400 for potassium and 600 calcium. (All values are extremely approximate.)
The graph of first ionization energy plotted against atomic number shows that ionization energy is a periodic function. First ionization energy generally increases from alkali metals to noble gases. Exceptions to this general trend can be seen in going from beryllium to boron and from magnesium to aluminum. These two deviations from the line can best be explained by considering each element's:
- electron configuration.
- atomic radius.
- nuclear binding energy.
- atomic mass.
correct response: a. this question requires the examinee to demonstrate knowledge of trends within periods and groups in the periodic table. the first ionization energy is the energy required to remove the first electron from an atom in its ground state. in the group 2 left paren roman numeral 2 a right paren elements, which include beryllium and magnesium, the electrons are configured such that there are paired valence electrons in an s orbital. group 13 left paren roman numeral 3 a right paren elements, which include boron and aluminum, have a single electron in the outermost p orbital. less energy is needed to remove a single electron from a p orbital than to remove an electron from a filled s orbital in the same energy level; therefore, group 13 left paren roman numeral 3 a right paren elements have lower first ionization energies than group 2 left paren roman numeral 2 a right paren elements.
Competency 0008
Apply knowledge of kinetic molecular theory, the nature of phase changes, and the
gas laws.
4. start bold Use the phase diagram below to answer the question that follows. end bold
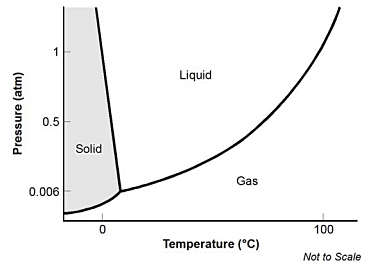
The phase diagram is a graph with a Y-shaped plot. The vertical axis is labeled Pressure in atmospheres, with values marked at 0.006, 0.5, and 1. The horizontal axis is labeled Temperature in degrees Celsius, with values marked at 0 and 100. The horizontal axis extends to the left of 0 degrees C, although no negative values are indicated. The data plot begins on the vertical axis somewhere below 0.006 and curves slightly upward to a pressure value of 0.006 atmospheres and a temperature value of about 10 degrees C. Two lines continue from that point. One goes steeply upward in a straight line, leaning slightly to the left, through a pressure value approaching 1.5 atmospheres and a temperature value of about 0 degrees C. The other curves smoothly upward to the right through a pressure value approaching 1.5 atmospheres and a temperature value of about 105 degrees C. The region of the graph between the vertical axis and the straight line and above the initial curve is shaded and labeled Solid. The region between the straight line and the other curve is labeled Liquid. The region between that curve and the horizontal axis is labeled Gas.
Which of the following statements is supported by the phase diagram?
- The substance sublimes at pressures above 0.006 atm.
- The substance's freezing point increases with increasing pressure.
- The substance's freezing point is between 0 degrees Celsius and minus 100 degrees Celsius at pressures below 1 atm.
- The substance occurs as a liquid at room temperature and pressure.
correct response: d. this question requires the examinee to demonstrate the ability to analyze phase diagrams. in most naturally occurring situations on the earth's surface, the pressure is approximately 1 atmosphere. at a room temperature of approximately 22 degrees celsius left paren 72 degrees fahrenheit right paren and a pressure of 1 atmosphere, the substance will be in a liquid phase.
Competency 0009
Apply knowledge of nuclear processes.
5.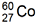 has a half-life of 5.27 years. How much of a sample with an original mass of 3.2 micro grams will remain after 21 years?
has a half-life of 5.27 years. How much of a sample with an original mass of 3.2 micro grams will remain after 21 years?
- 0.03 micro grams
- 0.20 micro grams
- 0.80 micro grams
- 1.60 micro grams
correct response: b. this question requires the examinee to demonstrate the ability to solve problems involving the half-life of radioactive materials. half-life is the length of time it takes half the mass of a sample of radioactive element to decompose. twenty-one years is equal to approximately four times the half-life of this element. thus, the sample is reduced first to one-half, then to one-quarter, then to one-eighth, and finally to one-sixteenth its original mass. the mass of sample remaining after 21 years or four half-lives can be calculated by multiplying the original mass by one-sixteenth left paren 3.2 micro grams times 1 sixteenth equals 0.20 micro grams right paren.
Competency 0011
Analyze energy changes in chemical bonding, chemical reactions, and physical processes.
6. start bold Use the information below to answer the question that follows. end bold
C H sub 4 left paren g right paren plus 202 left paren g right paren yields C O sub 2 left paren g right paren plus 2 H sub 2 O left paren l right paren delta H sub c equals negative 890.31 k J / mol
delta H sub f for C O sub 2 left paren g right paren is negative 393.51 k J / mol delta H sub f for H sub 2 O left paren l right paren is negative 285.81 k J / mol
The combustion of methane forms carbon dioxide and water as shown above. Which of the following expressions represents the heat of formation for methane?
- left square bracket negative 393.51 plus 2 left paren negative 285.81 right paren plus left paren negative 890.31 right paren right square bracket k J / mol
- left square bracket negative 890.31 plus left paren negative 393.51 right paren minus 2 left paren negative 285.81 right paren right square bracket k J / mol
- left square bracket negative 393.51 plus 2 left paren negative 285.81 right paren minus left paren negative 890.31 right paren right square bracket k J / mol
- left square bracket negative 890.31 minus left paren negative 393.51 right paren plus 2 left paren negative 285.81 right paren right square bracket k J / mol
Correct Response: C. This question requires the examinee to demonstrate the ability to solve problems involving energy changes during chemical reactions. The combustion of 1 mole of C H sub 4 produces 1 mole of C O sub 2 and 2 moles of H sub 2 O. The heat of formation of methane (C H sub 4) is equal to the sum of the heats of formation (delta H f) of the products minus the heat of combustion (delta H c) of methane. Thus the heat of formation of methane is equal to (delta H sub f C O sub 2 left paren g right paren plus 2 delta H sub f H sub 2 O left paren l right paren minus delta H sub c C H sub 4 left paren g right paren. The 2 moles of O sub 2 gas is omitted from the calculation because elements in their standard states have a heat of formation of zero.
Competency 0016
Analyze oxidation-reduction reactions and electrochemical processes.
7. Which of the following is an oxidation-reduction reaction?
- H C I left paren a q right paren plus H sub 2 O left paren l right paren yields H sub 3 O sup plus left paren a q right paren plus C l sup minus left paren a q right paren
- Na C l left paren a q right paren plus Ag N O sub 3 left paren a q right paren yields Ag C l left paren s right paren plus Na N O sub 3 left paren a q right paren
- 2 H N O sub 3 left paren a q right paren plus M g O left paren s right paren yields M g left paren N O sub 3 right paren sub 2 left paren a q right paren plus H sub 2 O left paren l right paren
- 2 H sub 2 O sub 2 left paren a q right paren yield 2 H sub 2 O left paren l right paren plus O sub 2 left paren g right paren
Correct Response: D. This question requires the examinee to demonstrate the ability to analyze processes that occur during oxidation-reduction reactions. An oxidation-reduction reaction is characterized by the transfer of electrons. By assigning oxidation numbers to the elements in the reactions, it can be seen that in the reaction 2 H sub 2 O sub 2 left paren aq right paren yields 2 H sub 2 O left paren l right paren plus O sub 2 left paren g right paren the oxidation number of oxygen changes from negative 1 in the H sub 2 O sub 2 molecule to negative 2 in the H sub 2 O molecule and 0 in the O sub 2 molecule, indicating a transfer of electrons. This reaction is the only one of those given in which the oxidation numbers of the elements change.
Competency 0018
Apply knowledge of solutions and suspensions.
8. Equal molar amounts of NaCl and CaCl2 are dissolved in two containers containing equal amounts of water. How will the freezing points in the two containers compare?
- Na C l and Ca C l sub 2 will lower the freezing point by the same amount.
- Na C l will lower the freezing point twice as much as Ca C l sub 2.
- Ca C l sub 2 will lower the freezing point three times as much as Na C l.
- Ca C l 2 will lower the freezing point one-and-a-half times as much as Na C l.
Correct Response: D. This question requires the examinee to demonstrate the ability to analyze the colligative properties of solutions. Freezing point depression in solutions is a colligative property that is dependent on the number of solute particles. In this problem, both Na C l and Ca C l sub 2 are electrolytes that dissociate into ions in solution, but each unit of Na C l dissociates into only two ions (one Na sup plus and one C l sup minus), while Ca C l sub 2 dissociates into three ions (one Ca sup 2 plus and two C l minus). Thus, a solution of Ca C l sub 2 will have one-and-a-half times the number of solute particles as an equal molar solution of Na C l. Since the decrease in freezing point of a solution is directly proportional to the concentration of solute particles, the freezing point of the Ca C l sub 2 solution will be depressed one-and-a-half times more than the freezing point of the Na C l solution.
start bold Use the diagram below to answer the two questions that follow. end bold
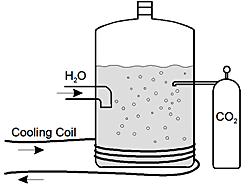
The diagram shows a large cylindrical vessel containing liquid with bubbles in it. A spigot through the left side of the vessel is labeled H sub 2 O with an arrow into the vessel. A labeled Cooling Coil enters the diagram from the left, wraps around the bottom of the vessel three times, and goes out of the diagram to the left. To the right of the vessel is a tank of gas, labeled C O sub 2, with a tube into the vessel.
In the beverage industry, carbon dioxide is introduced into a pressure vessel containing flavored sugar water to give the characteristic fizz associated with soda. After the system has reached equilibrium, the carbonated water is sent through tubing to be bottled.
Competency 0010
Apply knowledge of the principles of thermodynamics and calorimetry.
9. Which of the following pieces of information is needed in order to calculate the amount of energy required for the cooling coil to bring the contents of the vessel to the desired temperature?
- the volume of the vessel
- molar concentration of the sugar solution
- mass of the sugar water
- molecular weight of the sugar
correct response: c. this question requires the examinee to demonstrate the ability to analyze the three laws of thermodynamics and their application to chemical systems. the energy required to cool the contents of the vessel to the desired temperature can be calculated using the equation q equals m s delta t. in this equation, q represents the energy needed to cool the sugar water in the vessel, m is the mass of the sugar water, s is the specific heat of the sugar water, and delta t is the desired change in temperature. therefore, of the information provided, only the mass of the sugar water will be useful in calculating the energy needed to cool the contents of the vessel to the desired temperature.
Competency 0014
Apply knowledge of the principles of chemical equilibrium.
10. During the manufacturing process, which of the following conditions would shift the equilibrium to favor a reduced carbon dioxide concentration in the beverage?
- a leak in the pressure vessel
- a decrease in the temperature of the cooling coil
- an increase in the length of time the carbon dioxide is left in contact with the sugar water
- an increase in the level to which the vessel is filled with sugar water
correct response: a. this question requires the examinee to demonstrate the ability to analyze the effects of concentration, pressure, temperature, and catalysts on chemical equilibrium. several factors can affect chemical equilibrium, but in the situation described, temperature and pressure are likely to be of the greatest concern. either an increased temperature or a decreased pressure would be unfavorable to carbon dioxide going into solution. therefore, only a leak in the pressure vessel, which would lower the system's pressure, is likely to cause the beverage to have a reduced carbon dioxide concentration.
